Electrochemistry with MS detection
Reactors for EC/MS
The on-line coupling of electrochemistry (EC) with mass spectrometry (MS) has shown great potential for the investigation of drug metabolism in pharmaceuticals and for structure elucidation of biopharmaceuticals and proteins. As the title suggests, the technique consists of two parts. First, the electrochemical reactor where products or metabolites are formed. Second, the MS where the products are identified and in some cases quantified.

Fig. 1. A syringe pump and a potentiostat with an EC reactor inside.
Electrochemical reactor
In a flow-through electrochemical reactor a substrate solution is introduced by means of a syringe pump. The reactor is actually an electrochemical cell, it has a small potential applied over the electrodes to drive the reaction. The outlet of the cell is connected to an MS for analysis of the products.
There are several parameters that are important for a successful EC/MS experiment. The first is the applied potential, it supplies the energy to make a reaction happen. A positive potential for oxidation and negative for reduction reactions. Once the conditions are set and the reaction occurs, increasing the potential further will not speed up the reaction or generate more product. The reason for that is explained in traditional laws of electrochemistry. The limiting factor is not the potential, it is diffusion of substrate from the bulk solution to the electrode.
The second important parameter is the solvent composition. It can make a huge difference to have the correct pH or percentage organic solvent. For the reduction of proteins for example, formic acid is used to set the pH. But also because it triggers a reactive intermediate that catalyzes the reaction, described in more detail by B. Stocks and J. Melanson (2019).
Also, the flow rate is important, typically at lower flow rate a relative larger percentage of the substrate will be converted into product. The size of the electrodes and the spacing between electrodes are other factors that play a role.
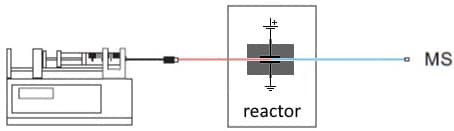
Fig. 2. Most basic configuration for EC/MS. A syringe pump supplies the substrate. In the EC reactor oxidation or reduction products are formed and analyzed in an MS.
Mass spectrometry
The reaction products are analyzed in the mass spectrometer. This on-line coupling has several benefits. First, it is really fast. Typically, within 15 seconds the reaction products are analyzed by MS. Second, the solvent is much cleaner than with alternative methods. Traditional methods often require addition of chemicals or enzymes, and a cleanup step is necessary before analysis in an MS. Third, with an on-line setup it is easier to automate a work-flow, or to use a make-up flow and add substrates or adjust the solvent pH and improve MS sensitivity.
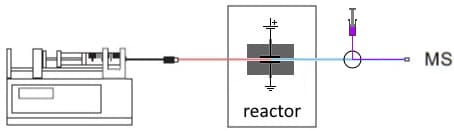
Fig. 3. On-line EC/MS configuration to study conjugation. A make-up flow with substrate is added after the reactor.
Pharmaceutical metabolite studies
On the right side an example is shown of EC/MS of amodiaquine (AQ). The m/z trace of AQ disappears and the metabolite traces appear when the reactor is switched on. Within 5 min all known metabolites of AQ are found and identified. More details are in our application note.
The technique offers fast electrochemical synthesis of drug metabolites for identification and quantification by mass spectrometry (MS). The technique can be operated in a flow-through configuration which makes it possible to implement in an automated work-flow or as a high throughput screening system.
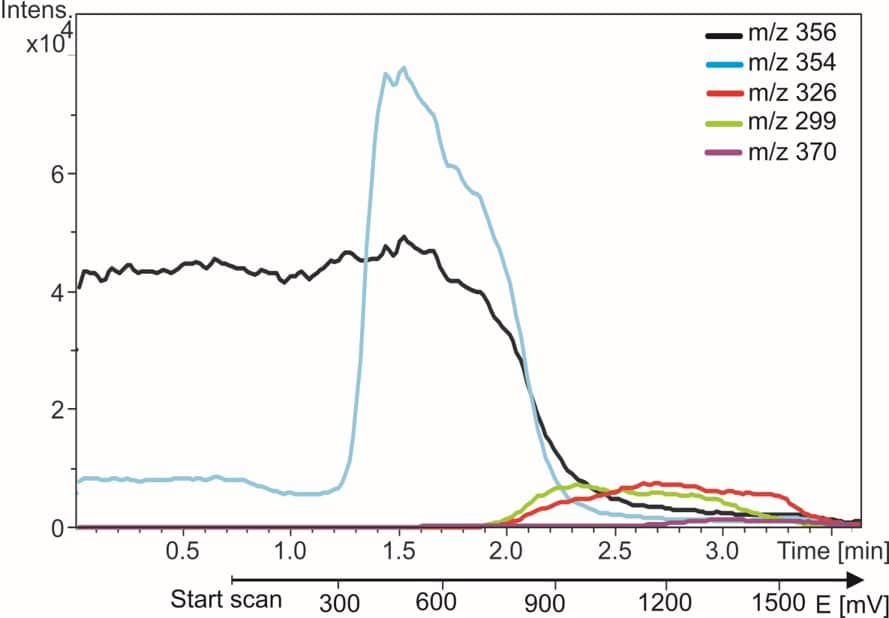
Fig. 3. Anti-malaria drug amodiaquine (black trace) and its oxidation products. The m/z traces are monitored while the reactor potential is incremented from 0 to 2 Volt.
Structure elucidation of proteins
Another application area of EC/MS is in the fast growing field of biopharmaceutical research. Biopharmaceuticals are medical drugs that are produced in living systems by using biotechnology. They are complex, high molecular weight proteins such as fusion proteins, bioaffinity proteins, antibodies (mAbs), and antibody–drug conjugates (ADCs).
With the growing number of biopharmaceuticals appearing on the market, there is a growing demand for efficient and automated strategies for structural elucidation. This can be quite a challenge. It is not only the primary structure, the mapping of amino acids that is important. It also concerns the higher order structure (HOS) defined by internal disulfide bridges and post translational modifications such as phosphorylations, acetylations, and glycosylations and so on. Structure is what defines the biological activity (review: D. Virág et al, 2020).
Disulfide bonds in proteins provide stability and folding, and are therefore of key importance for the biological functionality. In monoclonal antibodies for example, the disulfide bonds hold together the small and heavy chains. It is therefore important to verify the location and connectivity of disulfides in protein therapeutics. This is usually done by reducing the disulfide bonds and analyze the changes in the MS spectra (review: J. Lakudb et al, 2018).
Electrochemical reduction can automate such work-flow, by using an EC reactor in an on-line setup in connection with an MS. It is a small but significant step in the challenge for structure elucidation of biopharmaceuticals (C. Cramer at al, 2015).

Fig. 5. Disulfide bonds (yellow) play an important role in the structure of an antibody.

Fig. 6. Reduction (breaking) of a disulfide bond in a EC reactor at -1 V.
Applications
See also a video recording of the ASMS presentation of prof Uwe Karst (2010) and a computer animation comparing traditional vs EC/MS method for metabolite studies.