Disulfide bond reduction
ROXY Reactors for disulfide bond reduction
The ROXY Exceed with an EC Reactor has been developed for fast and efficient reduction of disulfide bonds in proteins. The reduction of intra- and intermolecular disulfide bonds is a small but crucial step in the workflow for successful structure elucidation and assignment of the bonding sites by MS. The method is based on an electric potential to trigger the reduction reaction, no reductive chemicals are used.
Traditionally, reduction is performed using concentrated chemicals, e.g. dithiothreitol (DTT) that needs to be removed prior LC/MS analysis. Alternatively, thiol – free reducing agents such as TCEP (tris (2-carboxyethyl) phosphine) can be used. However, sample preparation remains laborious and difficult to combine with on-line LC/MS. Electrochemistry in an on-line setup with MS is clean, no chemicals are needed and the result can be monitored instantaneously.
For more details: see our technology page.
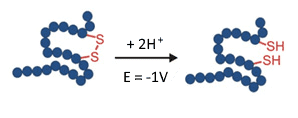
Breaking of a disulfide bond in an electrochemical flow-through reactor requires a potential of about -1 V.
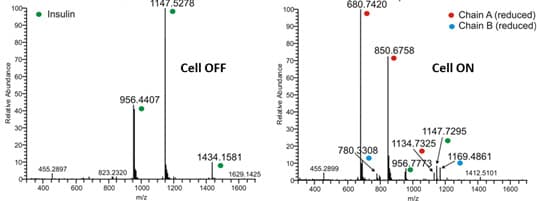
Insulin
Insulin, a small protein of 5733 Da is used as model compound for the S-S reduction. It consists of 51 amino acids forming two chains, A and B, and contains 3 disulfide bonds. Two interchain S-S bonds connecting chain A and B and one intrachain S-S bond located on chain A. The disulfide bonds can be completely reduced using an electrochemical reactor.
Electrochemical Reactors for MS and Synthesis
Environmental Degradation
Lipidomics, Lipid-Oxidation
Proteomics
Application notes
Posters
