Antibody Engineering
Antibody Engineering by Electrochemistry
- Electrochemical reduction, conjugation and re-oxidation of antibodies
- Green technology – electrons instead of toxic chemicals
- Selective – generate light and heavy chains
- Fast – seconds vs. hours with chemical approaches (e.g. TCEP, DTT)
This novel electrochemical approach provides a fast and efficient method for the selective reduction, conjugation and re-oxidation of antibodies without the need of reducing chemicals. The reduction of antibodies into its light chains (Lc) and heavy chains (Hc) is performed by the electrochemical method previously published, based on square-wave potential pulses applied to Antec’s proprietary Ti electrode. Subsequently, the free cysteine generated by the electrochemical reduction of the antibody can be conjugated. Finally, the free cysteine on both Lc and Hc can be very rapidly and selectively re-oxidized to reform the antibody in high yields. Electrochemical treatment did not affect the binding affinity of antibodies, while eliminating the need for harsh chemicals and additional tedious cleaning steps.
For more details: see also our technology page.
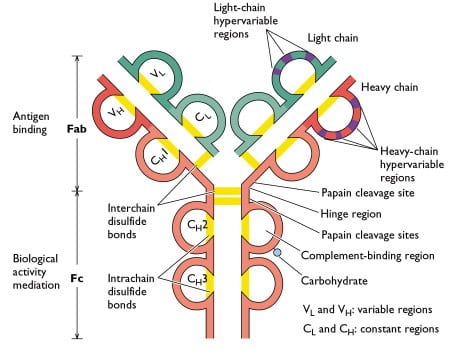
Graphical representation of an antibody with disulfide bonds holding the heavy and light chains together. Breaking of a disulfide bond in an electrochemical flow-through reactor requires a potential of about -1 V.
Mass Spectra
The electrochemical reduction of disulfide bonds is likely to prove itself as a major technological advancement not only in the analysis of antibodies but also in the design of novel antibody-based therapeutics, such as bi-specific antibodies or antibody-drug conjugates. The MS spectra below show the reaction products (Lc and Hc) after switching on the reactor cell (B and D).
Infusing an acidified antibody solution in the mass spectrometer with the μ-PrepCell turned OFF (A) resulted in a charge envelope spreading between m/z 2200 and 4000, with the intact antibody carrying approximately 40 to 60 charges. When the cell was turned ON (B), a shift of the antibody charge envelope toward lower m/z (~1000-2500) was observed.
The spectrum (C) results from the deconvolution of the MS signal (A) obtained with the cell OFF and confirms that the antibody is intact. D was obtained with the cell turned ON, and mostly shows species with molecular weights of approximately 25 and 50 kDa corresponding to the masses of Lc and Hc. The very low intensity of the MS signal for species with molecular weights greater than 50 kDa further confirms the selective reduction of the antibody inter-chain disulfide bonds in high yields.
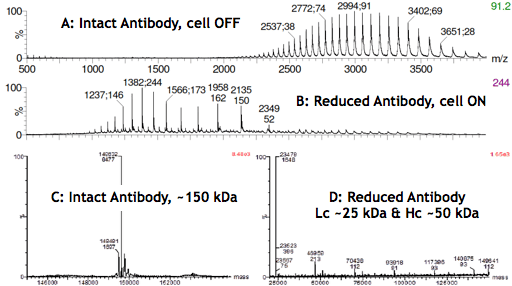
High-Resolution Mass Spectra of a Monoclonal Antibody before/after electrochemical reduction of the disulfide bonds.
ELISA binding assay
The curves obtained in ELISA for three different antibodies – in native form (green), electrochemically reduced and re-oxidized (red and blue) – show excellent agreement, illustrating that electrochemical treatment of antibodies does not affect their binding activity nor their biological activity, even when keeping the reduced antibody for several hours in acidic medium.
Courtesy: data kindly provided by Dr Xin Cheng & Arielle Verdi (Morphotek Inc., Exton, PA).

Evaluation of the ligand binding affinity by ELISA.
Electrochemical Reactors for MS and Synthesis
Environmental Degradation
Lipidomics, Lipid-Oxidation
Proteomics
Application notes
Posters