Disulfide Bond Reduction in proteins
ROXY Reactors for disulfide bond reduction
- Fast and mild reduction
- Replacing (harsh) wet chemistry
- No risk of ion suppression
- Superior S-S bond assignment
Disulfide bonds are one of the most important post-translational modifications of proteins. They are stabilizing the protein’s 3D structure and are crucial for their biological function. The reduction of intra- and intermolecular disulfide bonds is necessary for successful characterization and assignment of the bonding sites by MS. Off-line reduction is performed using highly concentrated chemical agents, e.g. dithiothreitol (DTT)) that needs to be removed prior LC/MS analysis. Alternatively, thiol – free reducing agents such as TCEP (tris (2-carboxyethyl) phosphine) can be used. However, sample preparation remains laborious and difficult to combine with on-line LC/MS. Moreover, the possibility of on-line disulfide bond reduction can be beneficial for the determination of disulfide bond arrangements or top down proteomics strategy, which relays on fragmentation of intact proteins without enzymatic digestion.
Finally, the use (LC)/EC/MS shows great potential for the fast assignment of S-S bonds in biopharmaceuticals. The ROXY EC and ROXY EC/LC system with its proprietary Titanium based electrodes are the ideal instruments to perform such studies.
Electrochemical Assisted Reduction of Disulfide Bonds in Insulin
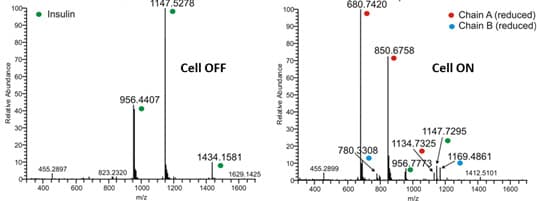
Insulin, a small protein of 5733 Da is used as model compound for the S-S reduction. It consists of 51 amino acids forming two chains, A and B, and contains 3 disulfide bonds. Two interchain S-S bonds connecting chain A and B and one intrachain S-S bond located on chain A.
Almost complete reduction of all three S-S bonds in Insulin was obtained using the ROXY EC system up-front MS.
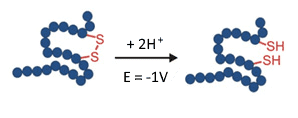
Breaking of a disulfide bond in an electrochemical flow-through reactor requires a potential of about -1 V.
[wpfd_category id=”25″]