Borate Ion Trap (BIT) column
- High borate trapping capacity
- Easy installation between pump and injector
- 4 x 50 mm column, 10 µm resin
In carbohydrate analysis, the peak shape of certain sugars, such as mannose, fructose and sugar alcohols, are deteriorated when traces of borate are in the solvent. These borate contaminants can come from the laboratory deionized water system. To eliminate the presence of borate ions and to assure optimal performance, Antec Scientific introduced the Borate Ion Trap (BIT) column. The trap column is available as 4.0 mm ID x 50 mm column and is installed inline between pump and injector of the HPAEC-PAD system.
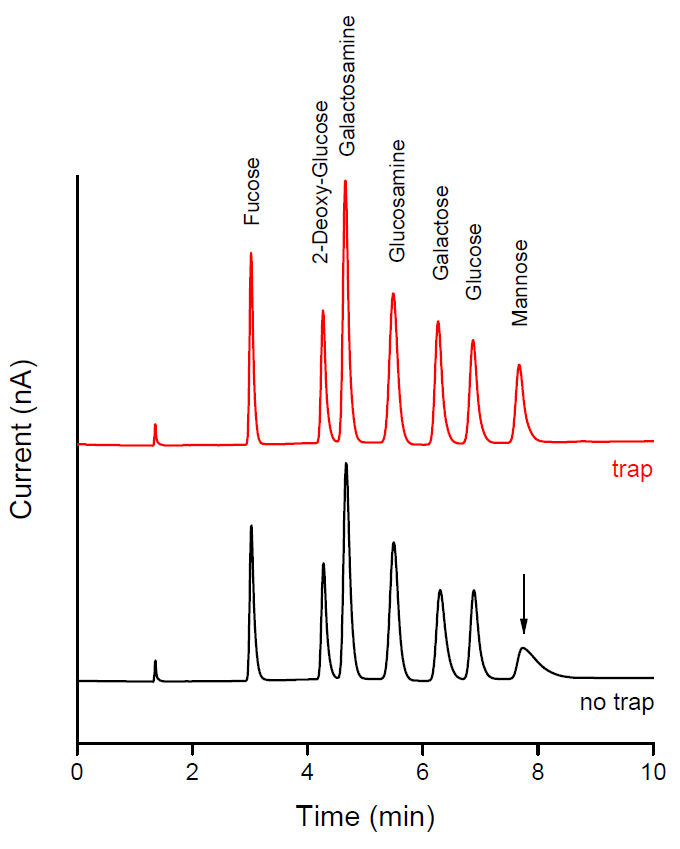
With and without BIT column. Without trap, the mannose peaks shows significant tailing by the presence of trace amounts of borate ions in the mobile phase, see lower trace with arrow.